Our key research theme is the rational design of non-viral gene therapy for serious diseases, inspired by the intrinsic nature of EV-mediated intercellular communication, with a particular focus on EVDNA. Our highly multidisciplinary and collaborative research spans pharmaceutical engineering, materials chemistry, EV biology, immunology, protein and genetic engineering, and data science. The current focuses of our group include:
Engineering Core-Shell Lipid Nanoparticles

Systemically administered LNPs mainly accumulate in the liver and are rapidly cleared by phagocytic cells due to the surface adsorption of serum proteins such as apolipoproteins, complements, and antibodies. Surface PEGylation creates a hydrophilic shell to reduce the formation of this ‘protein corona” and remains as the most widely used strategy. However, conventional LNPs can only tolerate less than 5 mol % surface PEGylation, inefficient for protecting them from serum protein binding. Our lab enginners unique core-supported LNP platforms based on lipid-polycation-nucleic acid nanoparticles or lipid-calcium-phosphate nanoparticles, allowing up to 30 mol% surface PEGylation. This provides greater flexibility to optimize targeting efficiency and pharmacokinetic profiles in vivo. We aim to establish a versatile core-shell LNP platform for targeted gene delivery to the site of interest extrahepatically or to specific cell types within the liver through further surface modification on the functionalized PEG.
Functional and Mechanistic Exploration of iEVs & iEVDNA
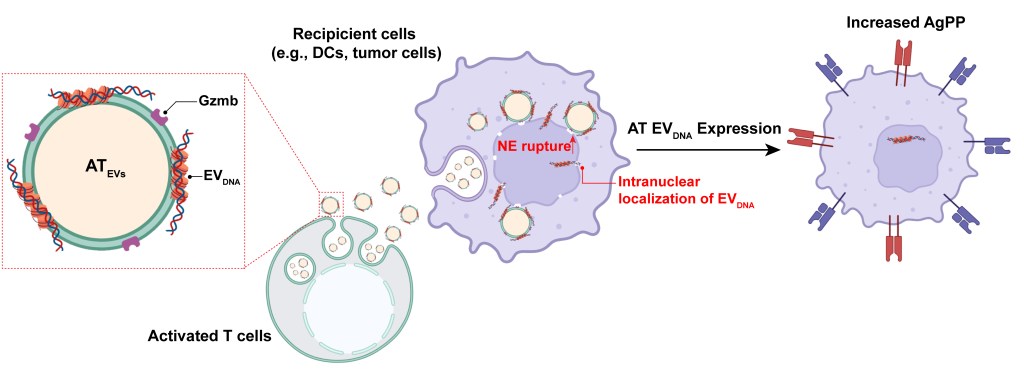
Extracellular vesicles (EVs) are naturally occurring cell-secreted particles that carry proteins, lipids, and genetic material from parental cells and can be taken up by recipient cells. Recognized as key mediators of intercellular communication, current EV studies predominantly focus on protein and RNA cargos. Our lab, however, is pioneering research into the function and underlying mechanisms of EVDNA. This focus stems from our discovery of 10-100-fold higher EVDNA secretion from specific immune cell types, including adoptive immune cells and neutrophils. For example, EVs secreted from activated T cells enhance the immunogenicity of immune-silent tumors. It is achieved by horizontally transferring EVDNA, enriched with antigen processing and presentation genes, into recipient dendritic cells and tumor cells. This process amplifies the magnitude and breadth of responses to immune checkpoint blockade therapies.
Our lab explores the role of EVDNA derived from T cells, B cells, and neutrophils, as well as the biogenesis of EVDNA in these immune cells across various diseases, including cancer, infectious and inflammatory diseases, and genetic disorders with the aim to inspire novel target discovery.
Therapeutics & Vaccines / Biomarkers Development
By integrating LNP-based gene delivery with a deep understanding of the functionality and mechanisms of iEVs and iEVDNA, we are interested to develop innovative therapeutics for cancer, infectious and inflammatory diseases, and genetic disorders. For instance, elucidating the physiological roles of T cell-derived EVDNA in immune homeostasis and responses to infections and cancer may pave the way for therapies that modulate EVDNA release from T cells. This could involve enhancing EVDNA release to boost immune responses against cancer or infections, or reducing it to mitigate T cell-mediated inflammation in autoimmune diseases. Activated T cell-derived EVs, as naturally occurring antigenicity-boosting gene therapy, can serve directly as an adjuvant for vaccines, eliciting potent disease-fighting immunity.
Moreover, we envision utilizing circulating EVDNA, which is larger (5-7 kb) and potentially richer in information compared to circulating cell-free DNA (160-180 bp), as a novel diagnostic and prognostic biomarker for patients.
